Subtotal $0.00
MINISTRY OF HEALTH AND FAMILY WELFARE
(Department of Health and Family Welfare)
NOTIFICATION
New Delhi, the 4th January, 2025
G.S.R. 10(E).—Whereas the Drugs Rules, 1945, were amended and published as required under sub-section (1) of section 12 and sub-section (1) of section 33 of the Drugs and Cosmetics Act, 1940 (23 of 1940), vide notification of the Government of India in the Ministry of Health and Family Welfare (Department of Health and Family Welfare) number G.S.R. 922 (E), dated the 28th December,2023, published in the Gazette of India Extraordinary, Part II, Section 3, Sub-section (i) dated the 5th January, 2024.
Whereas, the timeline of twelve months was provided for implementation of the provisions in respect of small and medium manufacturers having turnover of Rupees 250 crores or less which is expiring shortly.
And several representations have been received from various associations to extend this timeline to enable small and medium scale manufactures to comply with the provisions of revised Schedule M
Now, therefore, in exercise of the powers conferred by sections 12 and 13 of the Drugs and Cosmetics Act, 1940 (23 of 1940), with consideration that consultation with Drugs Technical Advisory Board shall be held as per the provisions, the Central Government intends to amend the notification number G.S.R. 922(E) dated the 28th December, 2023.
The draft notification is hereby published for information of all persons likely to be affected thereby, and notice is hereby given that said draft notification will be taken into consideration on or after the expiry of a period of seven days from the date on which copy of the Official Gazette of India containing the draft notification is made available to the public.
Objections and suggestions which may be received from any person within the aforesaid period will be considered by the Central Government. Objections and suggestions, if any, may be addressed to the Under Secretary (Drugs), Ministry of Health and Family Welfare, Government of India, Room No.414 A, D Wing, Nirman Bhawan,New Delhi – 110011 or e-mailed at drugsdiv-mohfw@nic.in;
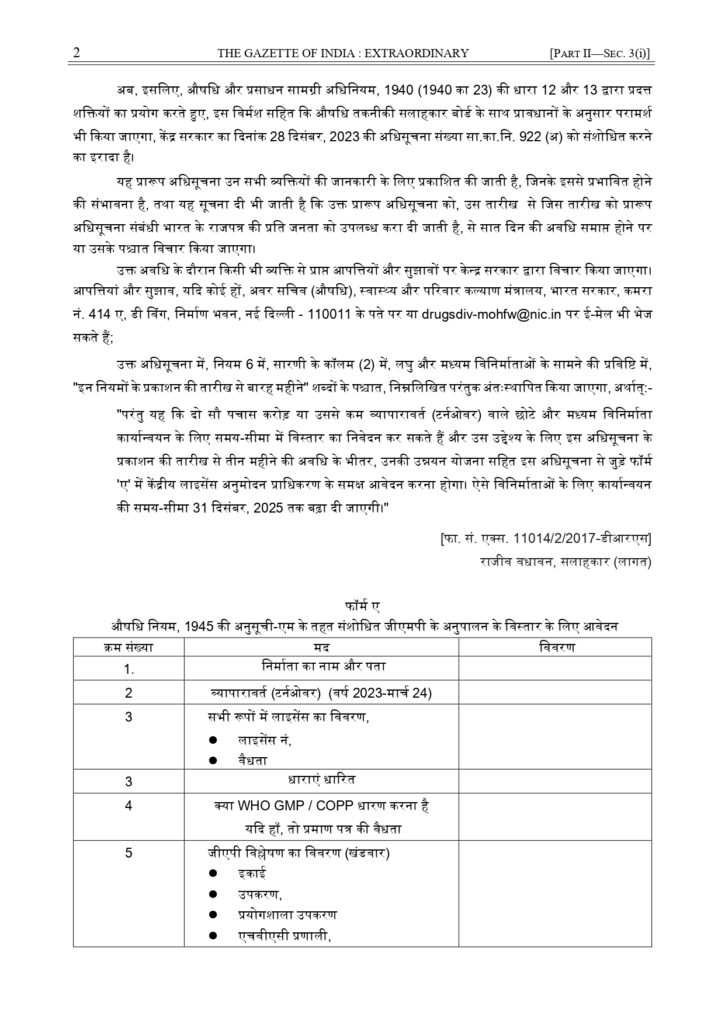
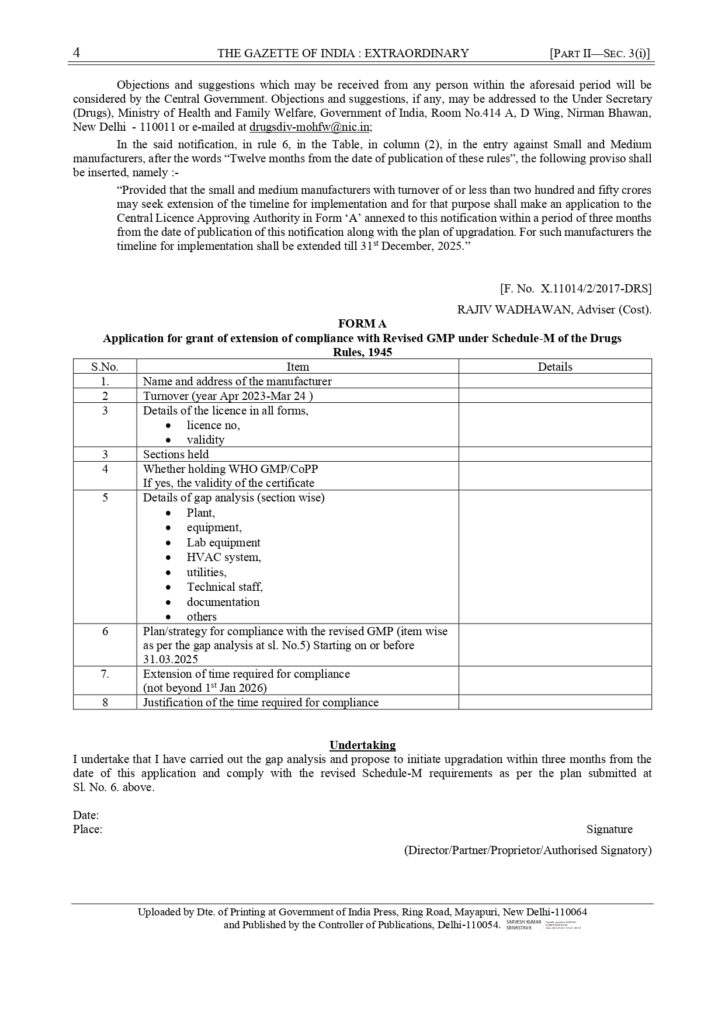
In the said notification, in rule 6, in the Table, in column (2), in the entry against Small and Medium manufacturers, after the words “Twelve months from the date of publication of these rules”, the following proviso shall be inserted, namely :-
“Provided that the small and medium manufacturers with turnover of or less than two hundred and fifty crores may seek extension of the timeline for implementation and for that purpose shall make an application to the Central Licence Approving Authority in Form ‘A’ annexed to this notification within a period of three months from the date of publication of this notification along with the plan of upgradation. For such manufacturers the timeline for implementation shall be extended till 31st December, 2025.”
[F. No. X.11014/2/2017-DRS]
RAJIV WADHAWAN, Adviser (Cost).